- Home
- Definition
- quantum event
WHAT IS A QUANTUM EVENT?
What is a quantum event? A quantum event is simply an event that occurs at the quantum level. In other words, it’s an event that occurs at the subatomic or atomic scales. Below are some examples of quantum events that occur at various times.
WHAT IS A QUANTUM EVENT: zeeman effect
The Zeeman effect is used to describe what happens when the hydrogen atom is placed in an external magnetic field. The presence of this field splits and diversifies the atom’s energy levels. There are three variations: the strong, weak, and intermediate effect.
As previously stated, the magnetic field splits the energy levels into different ones according to the magnetic field quantum number. To recount some background knowledge, in quantum physics there are 4 quantum numbers used to describe the state a particle is in. They are as follows:
principal quantum number-energy level of a quantum particle
azimuthal quantum number-indicates subshell in the specified energy level
magnetic quantum number-helps indicate the orbital angular momentum
spin quantum number- helps indicate the spin angular momentum
For our purposed we will assume that the particle undergoing the Zeeman effect has no spin (so no need to worry about the 4th quantum number 😊).
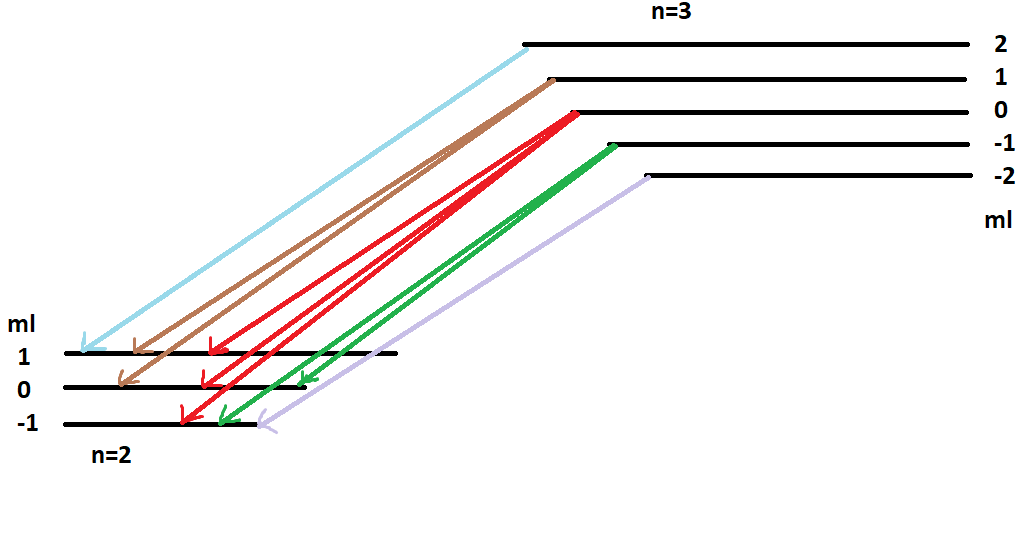
If you look in the below picture, the left diagram indicates electron transitions between energy levels in the absence of a magnetic field. The picture is pretty simple. The electron transitions between energy levels are represented by the principal number and there are only two levels: n=1 & n=2.
In contrast, the right diagram is in the presence of a magnetic field. The energies are split into different levels according to their magnetic quantum number. N=3 is split into 5 different levels because ml ranges from -2 to 2. N=2 is split into 3 different levels because ml goes from -1 to 1.
WHAT IS A QUANTUM EVENT: quantum tunneling
The next quantum event I will talk about is quantum
tunnelling. In classical mechanics, if an object, for example’s sake let’s say
a ball, encounters a 5 feet wall barrier we know from intuition and conventional
experience that the ball cannot pass the wall. However, in quantum mechanics a
situation analogous to this is possible. Let’s say an electron encounters a
very high potential (the higher the potential the harder it is for a quantum
particle to pass through it). If this happens there’s a small chance the
electron can slip through the potential barrier. I hope this increases your
understanding of quantum events. For more information read here.